Therapeutic Areas
Pulmonary
Administered via inhalation or intranasal instillation, siRNA can be deposited directly at the site of respiratory disease manifestation or viral replication. With intracellular delivery made possible by the MNM, siRNA can be used to treat life threatening disorders such as Cystic Fibrosis, and Idiopathic Pulmonary Fibrosis. Alternatively, the sequence of the siRNA can be tailored to target conserved genes of all 23 viruses that infect the human respiratory tract. Such an siRNA-based drug can be administered both before and after exposure to treat infection or prevent infection altogether. Such a treatment would be extremely fast acting, safe and simple to self-administer. At SirVir, an Aposense subsidiary, we are applying the Molecular Nano-Motors in order to overcome this hurdle. Clinical trials for our first viral indication are set to begin in Q1 2023, with additional antivirals entering clinical trials in late 2023 / early 2024.
Gastrointenstinal
Diseases of the gastrointestinal (GI) tract effect nearly 70 million individuals in the United States alone, causing more hospitalizations than any other family of disorders. The recent identification of target genes that contribute to some of the most serious GI diseases opens the door for therapies based on targeted and efficient gene down-regulation. Such therapies could be used to target mRNA coding for damaged or faulty proteins, or proinflammatory cytokines that drive inflammatory bowl diseases and other forms of mucosal inflammation. Were it possible to deliver siRNA into the cells lining the gut epithelium, it would be possible to treat diseases such as inflammatory bowel disease, celiac, and esophageal scarring. Aposense has demonstrated the ability of the MNM to mediate delivery of siRNA to target gut epithelial cells upon single oral administration in mice. Preclinical work continues with the goal of initiating clinical trials in 2024.
Central Nervous
System (CNS)
As our understanding of the pathogenesis of neurodegenerative disease deepens, it has become possible to identify specific genetic sequences whose active downregulation using gene therapy has the potential to treat diseases for which no treatments exist.
Direct administration into the spinal canal (Intrathecal administration) of MNM conjugated to siRNA provides a mode of direct of administration via which our revolutionary delivery technology can be brought to bear on these inherited neurological diseases.
Aposense is working in collaboration with its partners on tailoring the MNM platform to a range of severe and untreated heritable diseases of the nervous system.
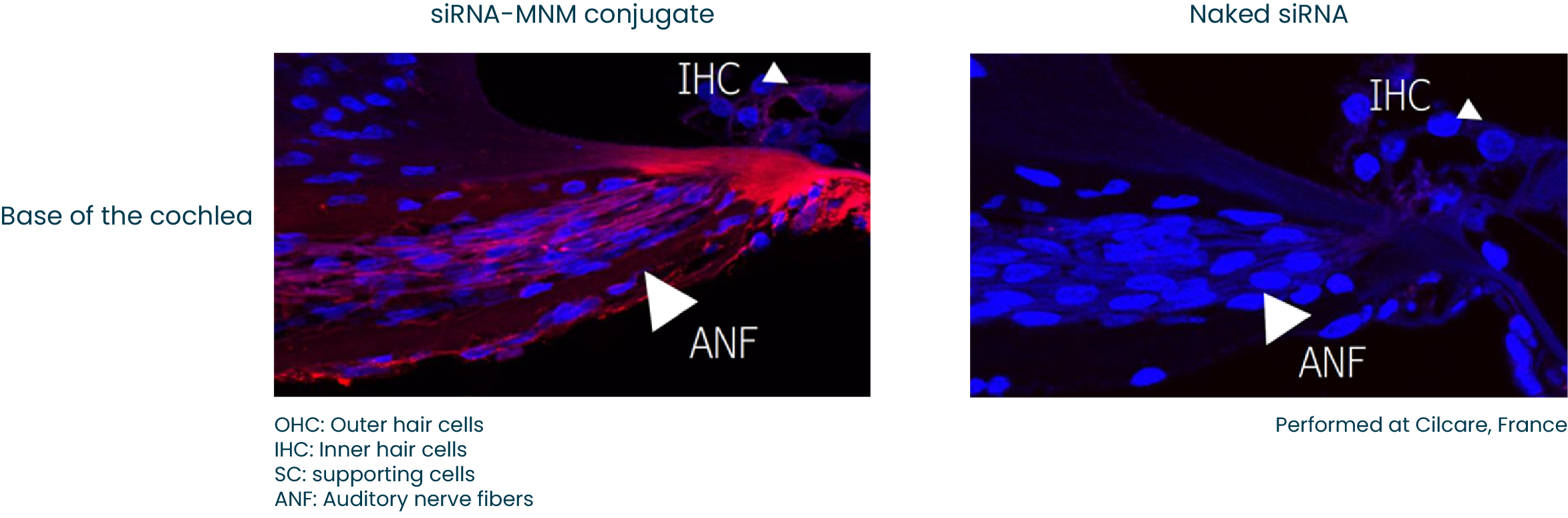
Inner Ear
The hearing disorders field represents a huge unmet medical need as it affects ~15% of the world’s adult population with no approved therapeutics. The physiological structure and conditions in the inner ear are favourable for our technology. To date we have shown that the Molecular Nano-Motors conjugate can effectively reach the auditory nerve fibers which resides in the inner ear. We are pursuing multiple hearing loss conditions. We anticipate moving our first clinical candidates hearing loss into clinical development in 2023