To set a novel, unified industry standard,
addressing the challenge of trans-membrane
delivery of genetic drugs for clinical use.
To set a novel, unified industry standard, addressing the challenge of trans-membrane delivery of genetic drugs for clinical use.
About
Aposense is a highly innovative Israeli biotechnology company with full capacity research facilities and scientific leadership, headed by the Professor Roger D Kornberg, Nobel Prize Laureate, 2006. Aposense developed a universal platform entitled Molecular Nano-Motors (MNMs) for the delivery of genetic drugs, such as siRNA, into cells based on a novel mechanism of action, utilizing membrane electrical forces.
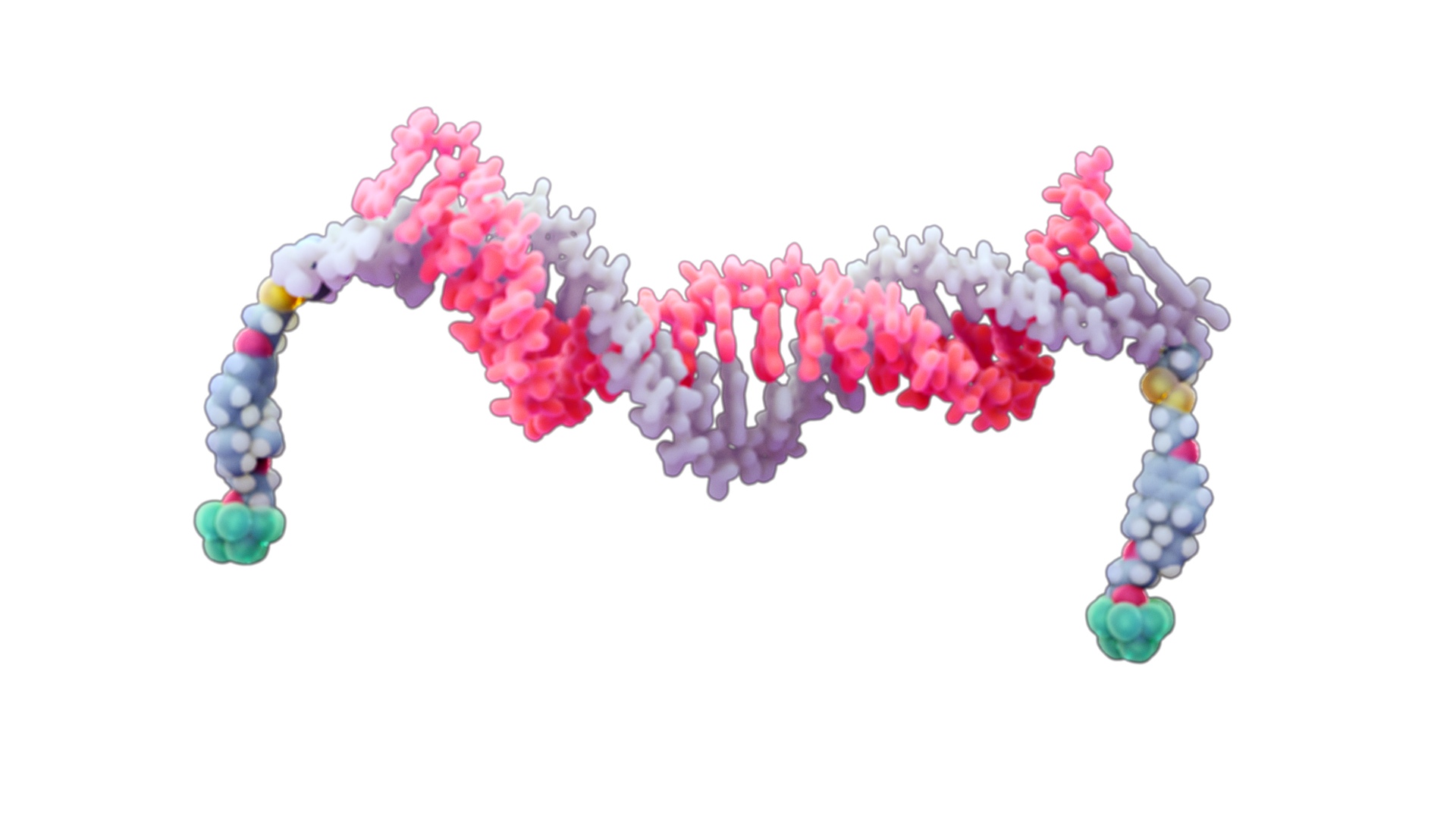
Our Technology
Therapeutic Areas
Platform technology extending widely beyond the Liver, unlocking novel targets for genetic therapeutics.
Platform technology with wide applicability in terms of therapeutic areas and genetic cargo.
Here are our prioritized therapeutic areas Aposense Molecular Nano Motors also has the potential to address additional therapeutic areas such as gastrointestinal, cardiovascular, eye, autoimmune.
These therapeutic areas are currently being investigated by Aposense’s team:
News
Aposense Receives Grant for Expanding its Molecular-Nano-Motors (MNMs) Delivery Platform to mRNA
Grant from Bill & Melinda Gates Foundation will support Development of Groundbreaking Macromolecule Drug Delivery Platform
SirVir obtains positive results in pre-clinical experiment against COVID-19
The English translation of an interview on Ynet with Prof. Roger Kornberg the scientific president of Aposense and SirVir. Petach Tikva, and Ness-Ziona, Israel. (April
