Our Proprietary Technology
Small interfering RNA (siRNA) therapies
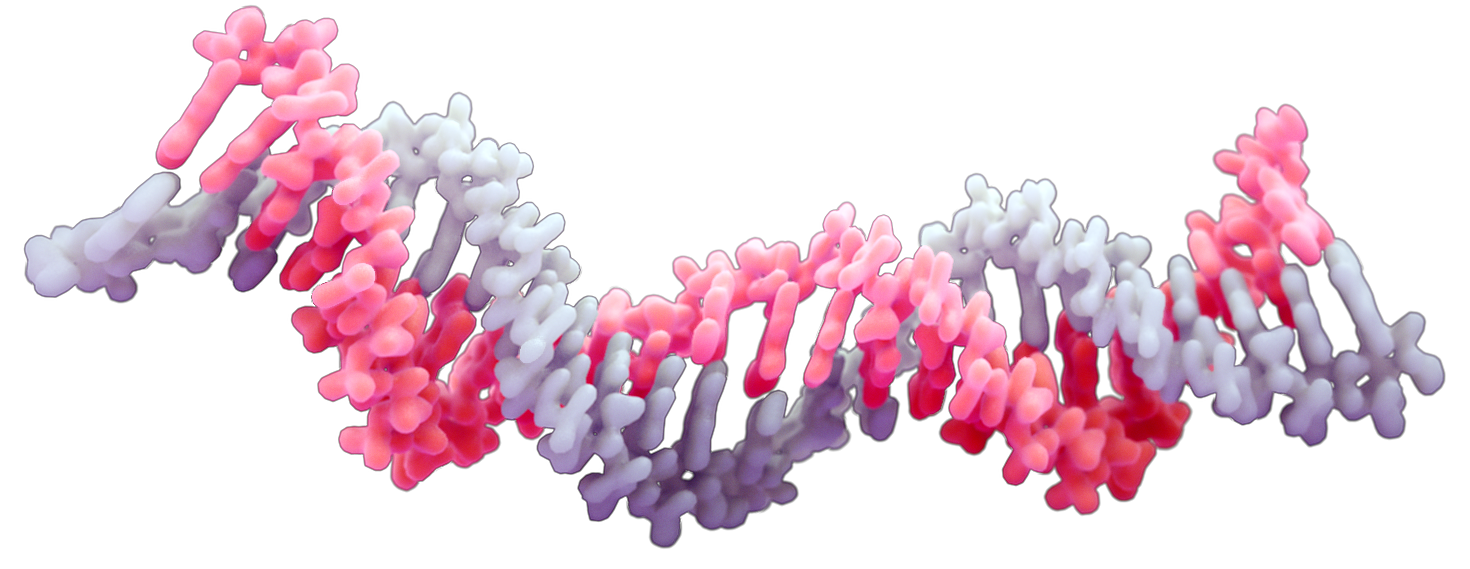
Small interfering RNA (siRNA) forms the basis of an adaptive defense mechanism used by human cells to selectively silence gene expression. Due to its high specificity and the catalytic nature of its mechanism of action (a small number of siRNA copies in a cell can result in very high levels of gene silencing), siRNA is regarded as a potentially potent therapeutic tool. Because the sequence of the siRNA can be readily tailored to match any gene sequence, there is potential for a wide range of therapeutic applications, ranging from Cancer to Cystic Fibrosis, Parkinson’s and Pandemic viral infection. In addition to its inherent specificity, the ability to easily tailor the therapeutic siRNA sequence means that future siRNA drugs will have the capacity to be rapidly edited to keep pace with diseases driven by mutagenesis, like cancer or viral infection. However, despite the great promise of siRNA-based therapy, and the significant resources that have been committed in efforts to bring such therapies into the clinic, to date only six siRNA-based drugs have been approved. The primary hurdle on the road to the widespread use of siRNA therapy is the difficulty in efficiently delivering siRNA into the cell cytoplasm.
Naked siRNA us unable to cross the cell membrane
The primary obstacle to the widespread use of siRNA therapy is the problem of delivering siRNA into the cell cytoplasm. The problem is the energetic barrier to the transport of large, charged molecules such as nucleic acids across the phospholipid bilayer of cell membranes. The energetic barrier is due to the low dielectric constant of the hydrocarbon interior of phospholipid bilayer membranes, which prevents the penetration of polar molecules, especially those bearing fixed negative charges.

MNM interacts with the cell membrane’s dipole potential
Aposense-SirVir has developed a unique delivery modality that harnesses the dipole potential present within all cell membranes. The potential is large (108-109 V/m), and can be leveraged for the delivery of genetic cargo. The Aposense-SirVir delivery modality exploits this universal property of phospholipid bilayer membranes for medical applications for the first time.
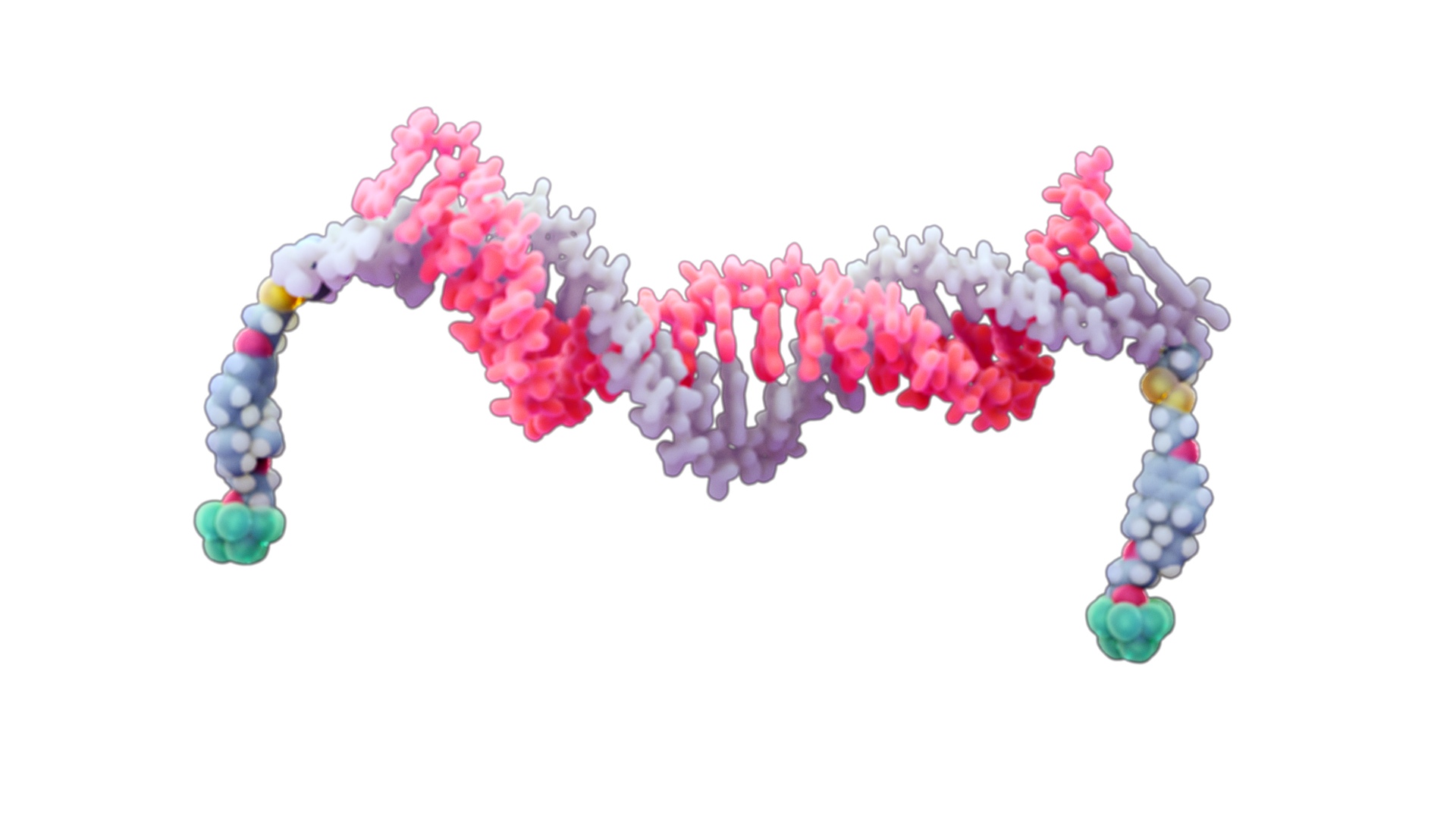
In the reductive environment of the
cytoplasm, the MNMs are detached
Two MNMs are attached to an siRNA through a linkage that is cleaved only upon entry into cells, thereby releasing the siRNA and trapping it in the cytoplasm. Once released, the siRNA can exert its biological effect, while the remnant MNM is metabolized and excreted.

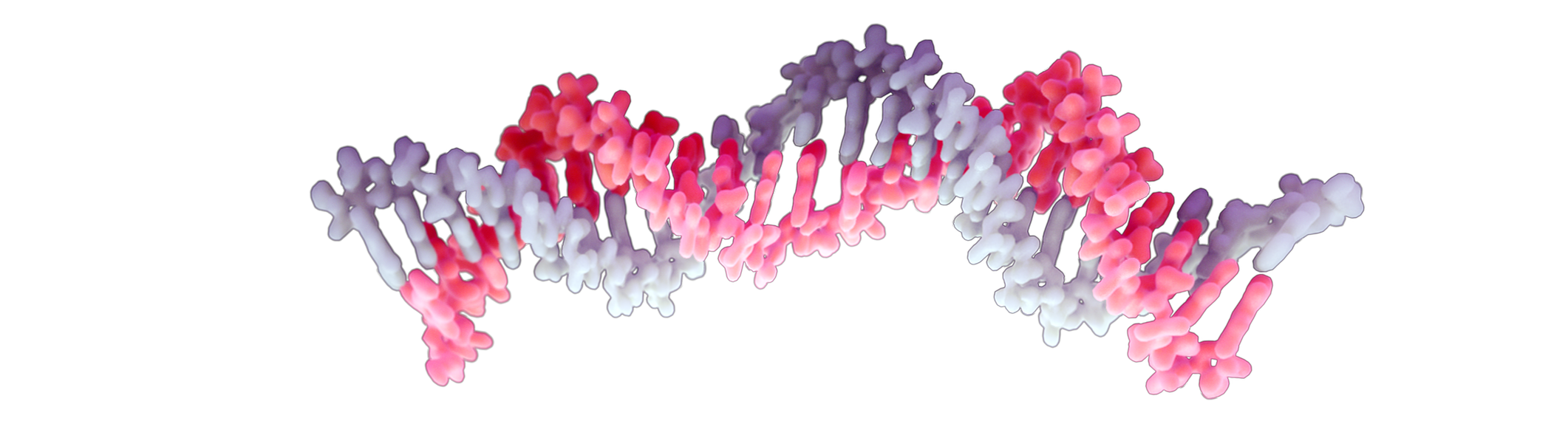
siRNA binds to RISC, directing target mRNA degradation
Once inside the cytoplasm, the siRNA guide strand associates with the RISC (RNA-induced silencing complex) machinery to direct targeted mRNA degradation. The siRNA-RISC complex can remain active for many days and even weeks, meaning that this therapeutic approach is targeted, potent and durable
Antiviral Platform Technology
The sequence of the siRNA can be tailored to target universally conserved viral genes, and can be rapidly modified to keep pace with mutagenesis. This same approach can be used to rapidly generate drug candidates for all 23 human respiratory viruses.
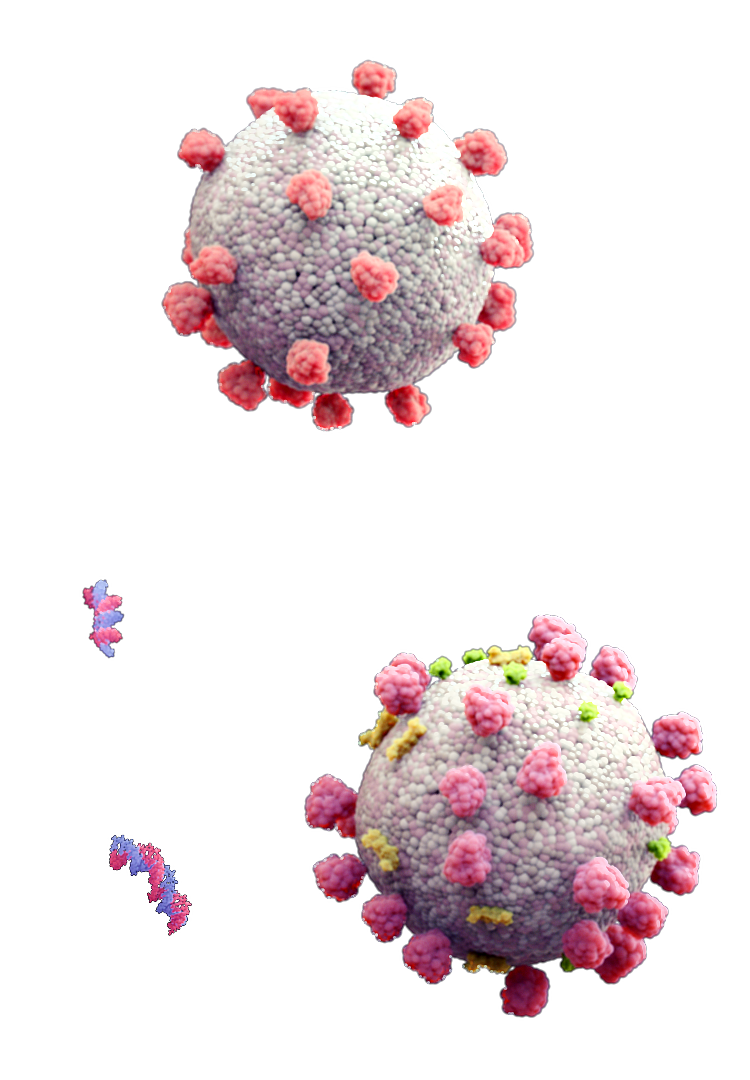


Universal Delivery Platform
Because the MNM achieves delivery via interaction with a universal feature of all cell membranes, this technology can be rapidly adapted for use in a range of therapeutic areas, and in a variety of different modes of administration.
